FDA approves first vaccine against RSV
Stephen Feller
Key takeaways:
- GSK’s vaccine against RSV was approved by the FDA and is expected to be available for people aged 60 and older this fall.
- A second RSV vaccine, from Pfizer, could also be approved by the FDA this month.
The FDA on Wednesday approved the world’s first vaccine against respiratory syncytial virus, which followed years of failed attempts by scientists to develop one.
The vaccine, GSK’s Arexvy, is approved in the United States to prevent lower respiratory tract disease caused by RSV in adults aged 60 years or older.
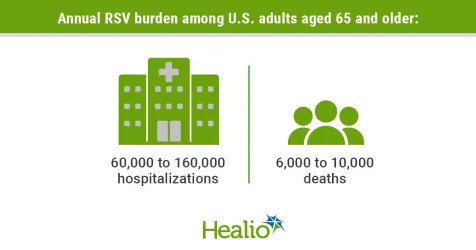
Data derived from CDC.
“Older adults, in particular those with underlying health conditions, such as heart or lung disease or weakened immune systems, are at high risk for severe disease caused by RSV,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said in a press release. “Today’s approval of the first RSV vaccine is an important public health achievement to prevent a disease which can be life-threatening.”
The FDA’s Vaccines and Related Biological Products Advisory Committee recommended the approval of GSK’s shot in March, in addition to a second RSV vaccine made by Pfizer. The FDA is expected to issue a decision on Pfizer’s vaccine at some point this month.
RSV is common, affecting people of all ages, but can cause serious infections, especially in older adults and infants. It kills up to 10,000 adults aged 65 years or older each year in the U.S., according to the CDC.
The U.S. experienced a surge in RSV this past winter — part of a “tripledemic” of respiratory infections that also included influenza and COVID-19, both vaccine-preventable diseases.
“We are pleased that we can now add a respiratory syncytial virus vaccine to providers’ options for patient care,” John Kennedy, MD, president of the American Medical Group Association, said in a press release issued by GSK.
The FDA’s approval was based on data from an ongoing randomized, placebo-controlled phase 3 trial, for which participants will remain enrolled for three RSV seasons to assess the duration of efficacy and safety of repeat vaccination.
In the trial, researchers found the vaccine reduced risk for developing RSV-related lower respiratory tract disease by 82.6% and reduced the risk for developing severe RSV-related lower respiratory tract disease by 94.1%.
The vaccine was generally well-tolerated by trial participants, with the most common side effects being injection site pain, fatigue, muscle pain, headache and joint stiffness or pain.
GSK is also conducting a clinical trial among people aged 50 to 59 years, including people with underlying comorbidities. It expects results from that trial sometime this year.
Results from two other trials testing coadministration with influenza vaccines are expected in the next several weeks, ahead of a June meeting of the CDC’s Advisory Committee on Immunization Practices, which will make recommendations on the use of the vaccine in the U.S. GSK said the vaccine should be available before the start of the 2023-2024 RSV season.
RSV vaccine research has ramped up over the past decade after a long delay in activity following a deadly vaccine trial in the 1960s.
References:
- FDA. FDA approves first respiratory syncytial virus (RSV) vaccine. https://www.fda.gov/news-events/press-announcements/fda-approves-first-respiratory-syncytial-virus-rsv-vaccine. Published May 3, 2023. Accessed May 3, 2023.
- US FDA approves GSK’s Arexvy, the world’s first respiratory syncytial virus vaccine for older adults. https://www.gsk.com/en-gb/media/press-releases/us-fda-approves-gsk-s-arexvy-the-world-s-first-respiratory-syncytial-virus-rsv-vaccine-for-older-adults/. Published May 3, 2023. Accessed May 3, 2023.
